- Specific Heat Capacity Calculator
- Specific Heat Capacity Bbc Bitesize
- Specific Latent Heat
- Specific Heat Capacity Of Aqueous Sodium Hydroxide
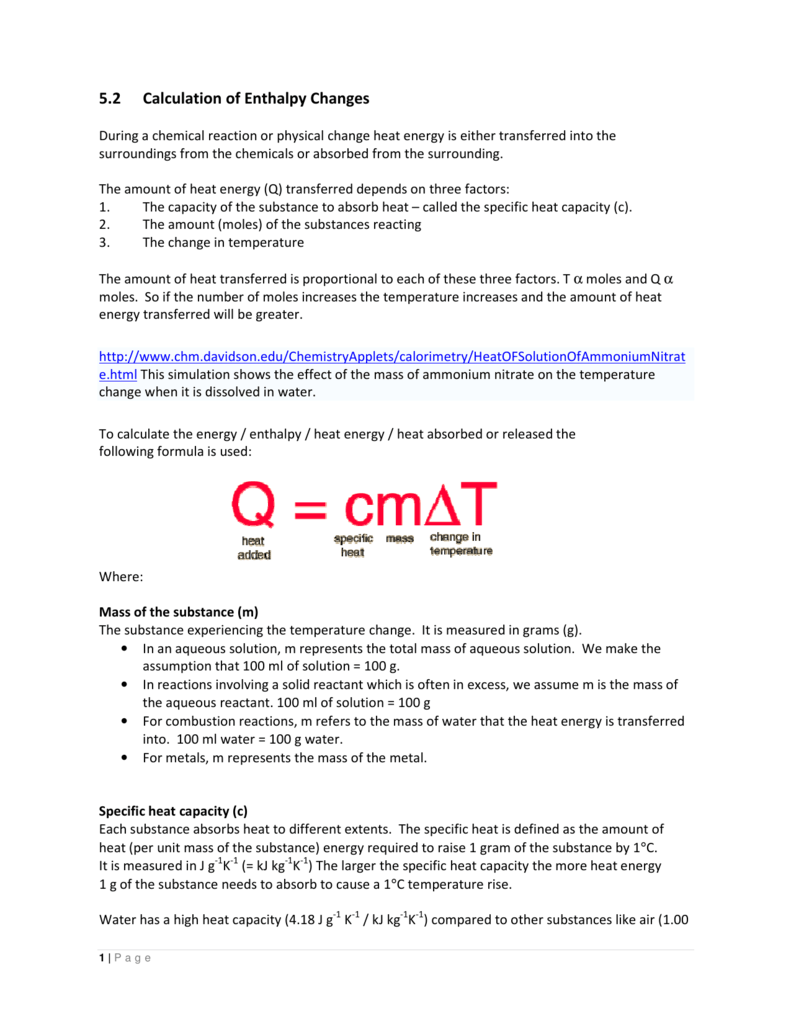
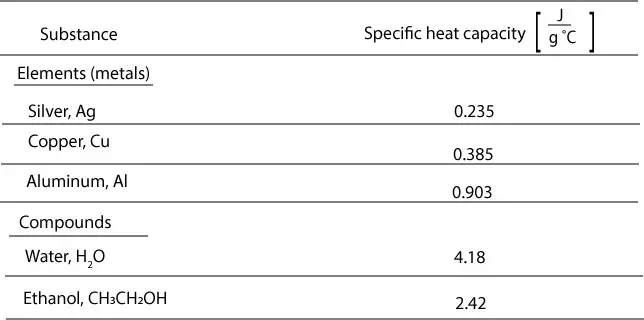
- Specific Heat Capacity Equation
- 50% Sodium Hydroxide Specific Heat Capacity
- Formula: HNaO
- Molecular weight: 39.9971
- IUPAC Standard InChI:
- InChI=1S/Na.H2O/h;1H2/q+1;/p-1
- Download the identifier in a file.
- IUPAC Standard InChIKey:HEMHJVSKTPXQMS-UHFFFAOYSA-M
- CAS Registry Number: 1310-73-2
- Chemical structure:
This structure is also available as a 2d Mol fileor as a computed3d SD file
The 3d structure may be viewed usingJavaorJavascript. - Species with the same structure:
- Information on this page:
- Data at other public NIST sites:
- Options:
SODIUM HYDROXIDE 32% W/W Specific hazards Corrosive liquid. Sodium hydroxide mists or vapours will be formed. Contact with metals may form hydrogen gas which is flammable and can result in explosion. Containers of flammable liquids in the area of the fire can explode upon heating. Hazardous combustion products. Specific Heat of HCl & NaOH Solution=4.017 J/g°C. Secondly, how do you calculate specific heat capacity? The units of specific heat capacity are J/(kg °C) or equivalently J/(kg K). The heat capacity and the specific heat are related by C=cm or c=C/m. The mass m, specific heat c, change in temperature ΔT, and heat added (or subtracted) Q are. SODIUM HYDROXIDE 32% W/W Specific hazards Corrosive liquid. Sodium hydroxide mists or vapours will be formed. Contact with metals may form hydrogen gas which is flammable and can result in explosion. Containers of flammable liquids in the area of the fire can explode upon heating. Hazardous combustion products.
Data at NIST subscription sites:
NIST subscription sites provide data under theNIST Standard ReferenceData Program, but require an annual fee to access.The purpose of the fee is to recover costs associatedwith the development of data collections included insuch sites. Your institution may already be a subscriber.Follow the links above to find out more about the datain these sites and their terms of usage.
Gas phase thermochemistry data
Go To:Top, Condensed phase thermochemistry data, Reaction thermochemistry data, Gas phase ion energetics data, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔfH°gas | -197.76 | kJ/mol | Review | Chase, 1998 | Data last reviewed in December, 1970 |
| Quantity | Value | Units | Method | Reference | Comment |
| S°gas,1 bar | 228.47 | J/mol*K | Review | Chase, 1998 | Data last reviewed in December, 1970 |
Gas Phase Heat Capacity (Shomate Equation)
Cp° = A + B*t + C*t2 + D*t3 + E/t2
H° − H°298.15= A*t + B*t2/2 + C*t3/3 + D*t4/4 − E/t + F − H
S° = A*ln(t) + B*t + C*t2/2 + D*t3/3 − E/(2*t2) + G
Cp = heat capacity (J/mol*K)
H° = standard enthalpy (kJ/mol)
S° = standard entropy (J/mol*K)
t = temperature (K) / 1000.
View plotRequires a JavaScript / HTML 5 canvas capable browser.
View table.
| Temperature (K) | 2500. - 6000. |
|---|---|
| A | 49.46492 |
| B | 7.000125 |
| C | -1.391757 |
| D | 0.095206 |
| E | -0.256928 |
| F | -213.6706 |
| G | 284.8609 |
| H | -197.7572 |
| Reference | Chase, 1998 |
| Comment | Data last reviewed in December, 1970 |
Condensed phase thermochemistry data
Go To:Top, Gas phase thermochemistry data, Reaction thermochemistry data, Gas phase ion energetics data, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔfH°liquid | -416.88 | kJ/mol | Review | Chase, 1998 | Data last reviewed in December, 1970 |
| Quantity | Value | Units | Method | Reference | Comment |
| S°liquid,1 bar | 75.91 | J/mol*K | Review | Chase, 1998 | Data last reviewed in December, 1970 |
| Quantity | Value | Units | Method | Reference | Comment |
| ΔfH°solid | -425.93 | kJ/mol | Review | Chase, 1998 | Data last reviewed in December, 1970 |
| Quantity | Value | Units | Method | Reference | Comment |
| S°solid | 64.46 | J/mol*K | Review | Chase, 1998 | Data last reviewed in December, 1970 |
Liquid Phase Heat Capacity (Shomate Equation)
Cp° = A + B*t + C*t2 + D*t3 + E/t2
H° − H°298.15= A*t + B*t2/2 + C*t3/3 + D*t4/4 − E/t + F − H
S° = A*ln(t) + B*t + C*t2/2 + D*t3/3 − E/(2*t2) + G
Cp = heat capacity (J/mol*K)
H° = standard enthalpy (kJ/mol)
S° = standard entropy (J/mol*K)
t = temperature (K) / 1000.
View plotRequires a JavaScript / HTML 5 canvas capable browser.
View table.
| Temperature (K) | 596. - 2500. |
|---|---|
| A | 88.34725 |
| B | -2.495103 |
| C | -3.013028 |
| D | 0.862607 |
| E | 0.042216 |
| F | -442.9350 |
| G | 183.9320 |
| H | -416.8783 |
| Reference | Chase, 1998 |
| Comment | Data last reviewed in December, 1970 |
Solid Phase Heat Capacity (Shomate Equation)
Cp° = A + B*t + C*t2 + D*t3 + E/t2
H° − H°298.15= A*t + B*t2/2 + C*t3/3 + D*t4/4 − E/t + F − H
S° = A*ln(t) + B*t + C*t2/2 + D*t3/3 − E/(2*t2) + G
Cp = heat capacity (J/mol*K)
H° = standard enthalpy (kJ/mol)
S° = standard entropy (J/mol*K)
t = temperature (K) / 1000.
View plotRequires a JavaScript / HTML 5 canvas capable browser.
View table.
| Temperature (K) | 298. - 572. | 572. - 596. |
|---|---|---|
| A | 419.4837 | 86.02304 |
| B | -1717.754 | 0.000000 |
| C | 2953.573 | 0.000000 |
| D | -1597.221 | 0.000000 |
| E | -6.046884 | 0.000000 |
| F | -517.8662 | -448.8512 |
| G | 933.0738 | 169.6281 |
| H | -425.9312 | -425.9312 |
| Reference | Chase, 1998 | Chase, 1998 |
| Comment | Data last reviewed in December, 1970 | Data last reviewed in December, 1970 |
Reaction thermochemistry data
Go To:Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Gas phase ion energetics data, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Data compiled as indicated in comments:
ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein
MS - José A. Martinho Simões
Note: Please consider using the reaction search for this species. This page allows searching of all reactions involving this species. A general reaction search form is also available. Future versions of this site may rely on reaction search pages in place of the enumerated reaction displays seen below.
Individual Reactions
Specific Heat Capacity Calculator
2 + = + +
By formula: 2HNaO + C2H3FO = C2H3NaO2 + FNa + H2O
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -56.15 ± 0.08 | kJ/mol | Cm | Pritchard and Skinner, 1950 | liquid phase; Corrected for CODATA value of ΔfH; HF(100); ALS |
| ΔrH° | -177. | kJ/mol | Cm | Carson and Skinner, 1949 | liquid phase; ALS |
2 + = + 2 + 2
By formula: 2HNaO + C2H6O4 = H2 + 2CHNaO2 + 2H2O
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -293.3 ± 5.0 | kJ/mol | Cm | Jenkins and Style, 1953 | solid phase; Reanalyzed by Cox and Pilcher, 1970, Original value = -292. kJ/mol; ALS |
+ =
By formula: HNaO + C2H2O = C2H3NaO2
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -208.2 ± 1.6 | kJ/mol | Cm | Nuttall, Laufer, et al., 1971 | gas phase; ALS |
| ΔrH° | -197.3 | kJ/mol | Cm | Rice and Greenberg, 1934 | gas phase; ALS |
C2Na2 (cr) + 2 (l) = 2( • 1418) (solution) + (g)
By formula: C2Na2 (cr) + 2H2O (l) = 2(HNaO • 1418H2O) (solution) + C2H2 (g)
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -161.8 ± 1.5 | kJ/mol | RSC | Johnson, van Deventer, et al., 1973 | Please also see Pedley and Rylance, 1977.; MS |
C2HNa (cr) + (l) = ( • 1418) (solution) + (g)
By formula: C2HNa (cr) + H2O (l) = (HNaO • 1418H2O) (solution) + C2H2 (g)
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -54.2 ± 0.8 | kJ/mol | RSC | Johnson, van Deventer, et al., 1973 | Please also see Pedley and Rylance, 1977.; MS |
3 + = CNa2O3 + + +
By formula: 3HNaO + C3H5ClO2 = CNa2O3 + C2H6O + ClNa + H2O
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -323.3 ± 1.7 | kJ/mol | Cm | Davies, Finch, et al., 1980 | liquid phase; Heat of hydrolysis; ALS |
2 + = + + CNO.Na
By formula: 2HNaO + CBrN = BrNa + H2O + CNO.Na
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -234.6 ± 0.71 | kJ/mol | Cm | Lord and Woolf, 1954 | solid phase; Heat of hydrolysis; ALS |
2 + = + + CNO.Na
By formula: 2HNaO + CIN = INa + H2O + CNO.Na
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -193.9 ± 0.3 | kJ/mol | Cm | Lord and Woolf, 1954 | solid phase; Heat of hydrolysis; ALS |
2 + = + + CNO.Na
By formula: 2HNaO + CClN = ClNa + H2O + CNO.Na
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -277.5 ± 0.4 | kJ/mol | Cm | Lord and Woolf, 1954 | solid phase; Heat of Hydrolysis; ALS |
+ = +
By formula: C2HBr3O + HNaO = CHNaO2 + CHBr3
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -93.72 | kJ/mol | Cm | Pritchard and Skinner, 1950, 2 | liquid phase; Heat of hydrolysis; ALS |
+ = +
By formula: HNaO + C2HCl3O = CHNaO2 + CHCl3
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -102.8 | kJ/mol | Cm | Pritchard and Skinner, 1950, 2 | liquid phase; Heat of hydrolysis; ALS |
+ = +
By formula: HNaO + C2H5NO = C2H3NaO2 + H3N
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -45.6 | kJ/mol | Cm | Calvet, 1933 | solid phase; Heat of hydrolysis; ALS |
C6H5NaO (cr) + (l) = (cr) + (cr)
By formula: C6H5NaO (cr) + H2O (l) = C6H6O (cr) + HNaO (cr)
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | 21.4 ± 3.6 | kJ/mol | RSC | Leal, Pires de Matos, et al., 1991 | MS |
C2H5NaO (cr) + (l) = (cr) + (l)
By formula: C2H5NaO (cr) + H2O (l) = HNaO (cr) + C2H6O (l)
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -5.7 ± 1.9 | kJ/mol | RSC | Leal, Pires de Matos, et al., 1991 | MS |
(cr) + (l) = (cr) + (l)
By formula: CH3NaO (cr) + H2O (l) = HNaO (cr) + CH4O (l)
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| ΔrH° | -6.5 ± 2.4 | kJ/mol | RSC | Leal, Pires de Matos, et al., 1991 | MS |
Gas phase ion energetics data
Go To:Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Specific Heat Capacity Bbc Bitesize
Data evaluated by:Edward P. Hunter and Sharon G. Lias
| Quantity | Value | Units | Method | Reference | Comment |
|---|---|---|---|---|---|
| Proton affinity (review) | 1071.8 | kJ/mol | N/A | Hunter and Lias, 1998 | |
| Quantity | Value | Units | Method | Reference | Comment |
| Gas basicity | 1044.8 | kJ/mol | N/A | Hunter and Lias, 1998 |
References
Go To:Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, Gas phase ion energetics data, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Chase, 1998
Chase, M.W., Jr.,NIST-JANAF Themochemical Tables, Fourth Edition,J. Phys. Chem. Ref. Data, Monograph 9, 1998, 1-1951. [all data]
Pritchard and Skinner, 1950
Pritchard, H.O.; Skinner, H.A.,The heat of hydrolysis of acetyl fluoride,J. Chem. Soc., 1950, 1099. [all data]
Carson and Skinner, 1949
Carson, A.S.; Skinner, H.A.,201. Carbon-halogen bond energies in the acetyl halides,J. Chem. Soc., 1949, 936-939. [all data]
Jenkins and Style, 1953
Jenkins, A.D.; Style, D.W.G.,The thermochemistry and pyrolysis of bishydroxymethyl,J. Chem. Soc., 1953, 2337-23. [all data]
Cox and Pilcher, 1970
Cox, J.D.; Pilcher, G.,Thermochemistry of Organic and Organometallic Compounds, Academic Press, New York, 1970, 1-636. [all data]
Nuttall, Laufer, et al., 1971
Nuttall, R.L.; Laufer, A.H.; Kilday, M.V.,The enthalpy of formation of ketene,J. Chem. Thermodyn., 1971, 3, 167-174. [all data]
Rice and Greenberg, 1934
Rice, F.O.; Greenberg, J.,Ketene. III. Heat of formation and heat of reaction with alcohols,J. Am. Chem. Soc., 1934, 38, 2268-2270. [all data]
Johnson, van Deventer, et al., 1973
Johnson, G.K.; van Deventer, E.H.; Ackerman, J.P.; Hubbard, W.N.; Osborne, D.W.; Flotow, H.L.,J. Chem. Thermodyn., 1973, 5, 57. [all data]
Pedley and Rylance, 1977
Pedley, J.B.; Rylance, J.,Computer Analysed Thermochemical Data: Organic and Organometallic Compounds, University of Sussex, Brigton, 1977. [all data]
Davies, Finch, et al., 1980
Davies, R.H.; Finch, A.; Gardner, P.J.,The standard enthalpy of formation of liquid and gaseous ethylchloroformate (C3H5O2Cl),J. Chem. Thermodyn., 1980, 12, 291-296. [all data]
Lord and Woolf, 1954
Lord, G.; Woolf, A.A.,The cyanogen halides. Part III. Their heats of formation and free energies,J. Chem. Soc., 1954, 2546-2551. [all data]
Pritchard and Skinner, 1950, 2
Pritchard, H.O.; Skinner, H.A.,The heats of hydrolysis of chloral and bromal, and the C-C bond dissociation energies in chloral and bromal,J. Am. Chem. Soc., 1950, 1928-1931. [all data]
Specific Latent Heat
Calvet, 1933
Calvet, E.,Mesures thermochimiques directes en chimie organique vitesses et chaleurs de saponification des amides. II.-Mesures effectuees et resultats obtenus,J. Chim. Phys., 1933, 30, 140-146. [all data]
Leal, Pires de Matos, et al., 1991
Leal, J.P.; Pires de Matos, A.; Martinho Simões, J.A.,J. Organometal. Chem., 1991, 403, 1. [all data]
Hunter and Lias, 1998
Hunter, E.P.; Lias, S.G.,Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update,J. Phys. Chem. Ref. Data, 1998, 27, 3, 413-656, https://doi.org/10.1063/1.556018. [all data]
Specific Heat Capacity Of Aqueous Sodium Hydroxide
Notes
Specific Heat Capacity Equation
Go To:Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Reaction thermochemistry data, Gas phase ion energetics data, References
50% Sodium Hydroxide Specific Heat Capacity
- Symbols used in this document:
S°gas,1 bar Entropy of gas at standard conditions (1 bar) S°liquid,1 bar Entropy of liquid at standard conditions (1 bar) S°solid Entropy of solid at standard conditions ΔfH°gas Enthalpy of formation of gas at standard conditions ΔfH°liquid Enthalpy of formation of liquid at standard conditions ΔfH°solid Enthalpy of formation of solid at standard conditions ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69:NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST)uses its best efforts to deliver a high quality copy of theDatabase and to verify that the data contained therein havebeen selected on the basis of sound scientific judgment.However, NIST makes no warranties to that effect, and NISTshall not be liable for any damage that may result fromerrors or omissions in the Database.
- Customer supportfor NIST Standard Reference Data products.